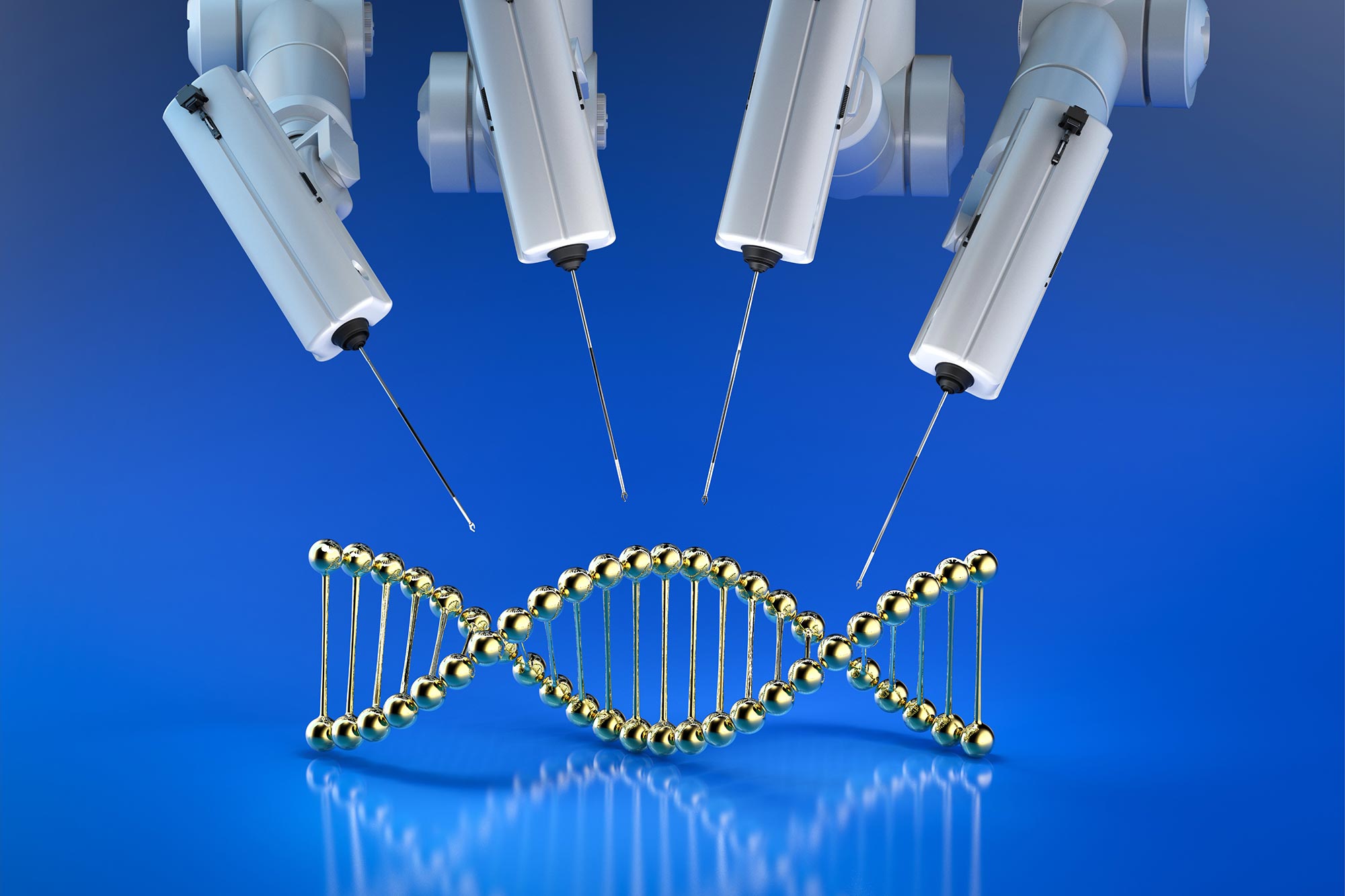
Investigadores de la Universidad de California, Santa Bárbara, han desarrollado un método para mejorar drásticamente la eficiencia de la edición de genes CRISPR/Cas9 sin utilizar material viral para entregar la plantilla del gen. método, como se describe en un artículo publicado en Naturaleza Biotecnología, utiliza enlaces cruzados para catalizar la reparación dirigida por homología, un paso en el proceso de edición de genes, aumentando la eficiencia tres veces sin aumentar las frecuencias de mutación. Se ha descubierto que estos enlaces cruzados, comúnmente utilizados en la quimioterapia contra el cáncer, mejoran los mecanismos de reparación naturales de las células y mejoran la probabilidad de modificar genes con éxito.
Los científicos triplicaron la eficiencia de la edición de genes CRISPR/Cas9 mediante enlaces cruzados, sin recurrir a material viral para la entrega. Este enfoque mejora los mecanismos de reparación naturales de la célula, lo que permite una edición de genes más precisa y eficiente, lo que podría mejorar la investigación de enfermedades y el trabajo preclínico.
La edición de genes es un método eficaz tanto para la investigación como para el tratamiento. Desde la llegada de la tecnología CRISPR/Cas9, ganadora del Premio Nobel, una herramienta de edición del genoma rápida y precisa descubierta en 2012, los científicos han estado trabajando para explorar sus capacidades y mejorar su rendimiento.
Investigadores del Laboratorio de Santa Bárbara de la Universidad de California, agregaron al biólogo Chris Richardson a esta creciente caja de herramientas, de una manera de aumentar la eficiencia de la edición CRISPR/Cas9 sin usar material viral para entregar la plantilla genética utilizada para editar la secuencia del gen objetivo. Según su nuevo artículo publicado en la revista Naturaleza BiotecnologíaSu método induce la reparación dirigida por homología (un paso en el proceso de edición de genes) casi tres veces «sin aumentar las frecuencias de mutación ni alterar el resultado de la reparación del acoplamiento terminal».
«Hemos encontrado una modificación química que mejora la edición de genes no virales y también hemos descubierto un nuevo tipo interesante de[{» attribute=»»>DNA repair,” Richardson said.
Find, Cut and Paste
The CRISPR/Cas9 method works by capitalizing on a defense technique employed by bacteria against viral attackers. To do this, the bacteria snip a piece of the invading virus’s genetic material, and incorporate it into their own in order to recognize it later. Should the bacteria get reinfected, they can target the now-familiar genetic sequences for destruction.
In gene editing, this process uses the enzyme Cas9 as molecular “scissors” to snip sequences it recognizes, guided by the CRISPR system. This cut is also an opportunity to replace the severed genes with similar (homologous) but improved ones, utilizing the cell’s natural repair mechanisms. If successful, the cell should have modified expressions and functions thereafter.
To deliver the repair template DNA to the nucleus of the cell where its genetic material lives, oftentimes viruses are used. While they are effective, the researchers say, viral workflows “are expensive, difficult to scale and potentially toxic to cells.”
Nonviral templates are potentially less expensive and more scalable, although researchers still must overcome efficiency and toxicity barriers. In their study, the Richardson Lab found that introducing interstrand crosslinks into the workflow increased homology directed repair dramatically.
“Every workflow that we have put this approach into has worked better by roughly threefold,” Richardson said.
Interstrand crosslinks are lesions that keep the double strands of a DNA helix tethered to each other, making them unable to replicate. Cancer chemotherapies use this mechanism to interrupt tumor growth and kill cancer cells. Added to a homology directed repair template, however, these crosslinks were found to stimulate the cell’s natural repair mechanisms and increase the likelihood of editing success.
“Basically, what we’ve done is taken this template DNA and damaged it,” Richardson said. “We’ve in fact damaged it in the most severe way I can think of. And the cell doesn’t say, ‘Hey this is junk; let me throw it away.’ What the cell actually says is, ‘Hey this looks great; let me stick it into my genome.’” The result is a highly efficient and minimally error-prone nonviral system of gene editing.
Their discovery, like many breakthroughs in science, was actually something of a happy accident. While working to purify proteins to study DNA repair, graduate student researcher and lead author Hannah Ghasemi noted unanticipated changes to the outcomes of their experiments.
“We were introducing these chemical modifications to the DNA templates in order to be able to pull them out of the cells and see what proteins were bound to them, and I was just checking to see if this modification had somehow affected the editing in any capacity,” she said. “I was expecting to either see no change or that it actually might have negatively affected the editing.”
What she found instead was a positive effect, up to three times the editing activity of the uncrosslinked controls. Furthermore, the team found that even with the increase in edits — and therefore the chances for errors — there was no increase in mutation frequency. They are still investigating the specific mechanisms leading to this result, but they have ideas.
“What we think happens is that the cell detects and tries to repair the damaged DNA that we’ve added this crosslink to,” Richardson said. “And in doing so, it delays the cell past a checkpoint where it would normally stop this recombination process. And so by prolonging the amount of time that it takes the cell to do this recombination, it makes it more likely that the edits will go to completion.” Studying this new process could also lead to a better understanding about how cells detect editing reagents and how they “decide” to accept them or not, he said.
This method will find the most use in ex-vivo gene editing applications, according to the team, that is, in the realm of disease research and preclinical work.
“We can more effectively knock down genes and insert things into genomes to study systems outside of the human body in a lab setting,” Ghasemi said. This development allows them to more efficiently build disease models and test hypotheses about how diseases work, which could lead to better clinical and therapeutic approaches.
Reference: “Interstrand crosslinking of homologous repair template DNA enhances gene editing in human cells” by Hannah I. Ghasemi, Julien Bacal, Amanda C. Yoon, Katherine U. Tavasoli, Carmen Cruz, Jonathan T. Vu, Brooke M. Gardner and Chris D. Richardson, 27 February 2023, Nature Biotechnology.
DOI: 10.1038/s41587-022-01654-y

«Organizador aficionado. Aspirante a evangelista de la cerveza. Fanático de la web en general. Ninja certificado de Internet. Lector ávido».




More Stories
¿Qué pasó cuando una roca del tamaño de Londres chocó contra la Tierra?
El espermatozoide no puede abrir el óvulo sin esta antigua clave molecular
Cuándo y dónde verla el lunes con «Shooting Stars»